'New chapter' opens for France's Carmat as EU approves artificial heart sales
Artificial heartmaker Carmat will begin sales of its devices from the second quarter of this year after a long-awaited European Commission approval, in a boost to its shares.
Artificial heartmaker Carmat will begin sales of its devices from the second quarter of this year after a long-awaited European Commission approval, in a boost to its shares.
Given recurring shortages of donors, Carmat's device aims to give patients with end-stage biventricular heart failure, a deadly condition where the heart is no longer able to pump blood adequately around the body, an alternative to hospital stays.
"It (EU approval for commercialisation of Carmat artificial heart) is an important phase... it's a new chapter for Carmat," CEO Stephane Piat told Reuters on Monday (January 11)
Although France's Carmat has yet to generate any significant revenue, its devices could represent a major breakthrough as heart diseases represent a leading cause of death worldwide.
Last month, Carmat secured European regulatory approval for its product in a so-called "bridge-to-transplant," although the European Commission however did not approve its artificial heart as a permanent implant.
Several estimates put at around 2,000 the number of patients in France, Germany, Italy, Spain and Britain with this specific condition on waiting lists for a heart.
Piat said the company is striving to have an extension of the EU approval so that it can cater to people who, because of pre-existing conditions, could not be considered into the waiting list for a heart transplant.
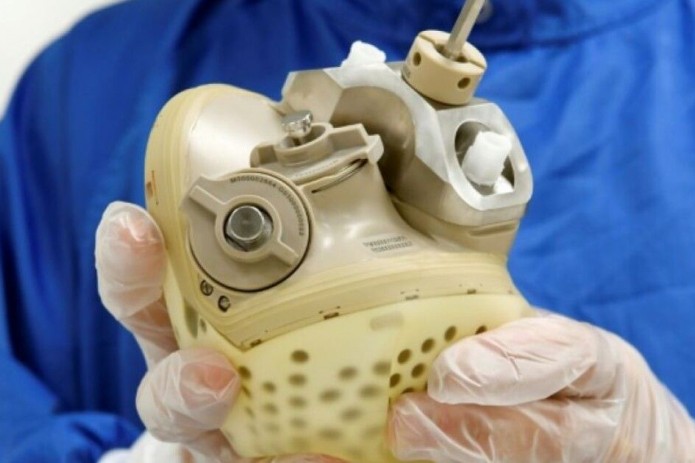
Carmat's bioprosthetic device, baptised Aeson, is designed to replace the real heart for "years," Piat said, mimicking nature's work using biological materials and sensors.
Composed of two ventricular cavities and a biomembrane, the battery-powered material weighs 900 grammes, while a human heart weighs 300 grammes.
Piat said Carmat aims to manufacture 10 artificial hearts monthly in 2021, a rate that could increase depending on demand.
In Carmat's home city of Velizy-Villacoublay, outside Paris, staff on Monday continued to refine the device ahead of its sale. Twelve machines, filled with saline solution that resemble human blood's temperature and salinity and powered by a pump, tested one of the artificial hearts.
For Carmat, a challenge, though, lies ahead with reimbursement by social welfare systems for the device which price per unit, Piat said, will top 150,000 euros.
Carmat, which has suffered setbacks with the death of patients in past trials, is conducting several studies to convince governments to buy its product.
Asked whether the device is safe, Piat said patients implanted with it in early trials faced more risks than today but that further studies have lowered those risks. Concerning surgery risks, the device has had a 100 percent success rate in trials, Piat said, adding that the "product's reliability is very high."
Carmat is also launching a study in the United States this quarter with the hope of getting approval from the Food and Drug Administration for its device by 2024.
Piat said the Carmat heart proves that innovation has no limits.
"If we can create a heart, we can create a lot of other things," he said. "The only limit is our imagination."





.png)



















